How bad is it to undervoltage a 12-volt lead-acid battery?
Your point can be very easily made differently.
If you look at the discharge curve for a Lead-Acid Battery with a 12V or 6V rating:
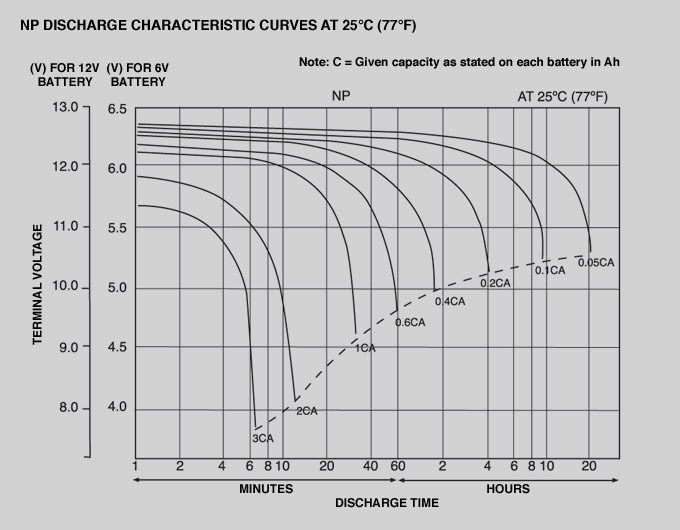
This comes from Yuasa. They make the things. It's either reliable or optimistic, certainly not pessimistic.
Let's look at the 12V one and optimistically assume that you are only interested in 0.2C discharge, any other rate the same arguments can be made with a different line.
At its 12V mark, you can see there is a "tipping point" where the voltage goes from relatively constant to plummeting.
At 11V it is almost going straight down.
At 9V it will drop right away under the same loading current.
This means that at the 12V point you have essentially used 60% of its capacity. At 11V you are at effectively 93%. At 9V you're at 99.5%.
Now adding that a battery with 0V across it has no chemical initiative any more, of any chemistry type, you will need to motivate it re-create the chemical imbalance that causes the apparent voltage and potential for current to flow. This is hard in any battery (which is why almost all batteries are built with electrodes already chemically built of the right materials to create the cell potential).
With SLA a lot of energy goes in chemical recombination of Sulphates and Sulphites, wasting a lot of energy. This results in needing excessive power to re-engage a cell that is left at 0V. Excessive power = heat. Heat = gassing. Gassing = moisture loss. Moisture loss = bad. Not to mention the higher voltage usually required makes many, many by-products on the plates, next to by-products already generated by neutering it in the first place and you're left with a AA battery with the weight and size of a 6Ah SLA.
Now, if you go near 9V, it will become 0V very quickly. You'll easily be too late.
Many manufacturers tell us "Consider your battery empty at 11.8V", some people (me included) assume 11V. Those who use 11V often (but not always) take care to know that this is the lower limit. This is exactly for that reason.
If they will not accept that 100's of manufacturers, countless experts in the field and users alike say "at 11.8V you're not going to get much more out of it and it'll be risky to try", then just convince them with the fact that at 11V there won't be much to get any more anyway. Done. All other points moot.
The reason a car battery can be dropped to 2V and then keep working, is because that battery was at 2V very shortly, because the idiot leaving his lights on realised after a while. And because they are usually over dimensioned by a factor of 2 to 5, depending on the type and brand of a car, so a crippled one will work for a couple more years.
And let's be honest, are we really going to put hours and hours of work and research into convincing someone who can't even manage to mind his car lights of agreed fact shared between thousands of electrical engineers worldwide?
Excellent answer from Asmyldof. The only things I'd add is that:
(1) There are several distinct varieties of lead-acid: the 'starter battery' that's intended to very rarely be discharged very far, the 'motive battery' intended for gradual & deeper discharge, the 'standby battery' for UPS style operation where deep discharges are rare and so the cumulative negative impacts of such deep discharge is offset by the expected lifetime, and a new flavour that can operate in a partial state of discharge for long periods. They all have quite distinct characteristics in the fine detail.
(2) The lead-acid battery industry is very competitive. Unless you've got some new patented chemistry to set you apart from the competition, then (a) you get what you pay for (in terms of quality), and (b) no manufacturer benefits from stating usage limits / recommendations below what is reasonable to expect a good service life from their products.
Knuckle-draggers who say "Oh these things are bullet-proof", or "I ran this b**ch down to 2.5V and it still works fine!" usually aren't the ones who have to cough up hundreds/thousands of their own $ when the batteries "mysteriously" don't last the several years that they should. These are the people who are also responsible for batteries having short warranties, because it can be difficult to prove that a customer mis-used a battery outside of published limits (without having elaborate monitoring electronics built in to record & prove it).
The manufacturer's datasheet is king, and your application must be matched with a battery intended for that use case. Your colleague's three datapoints of personal experience are worthless, at least in the context of battery longevity (& who has to pay for premature replacement).
A major factor to consider with multi-cell batteries is that damage caused by under-voltage will be concentrated on the weakest cell, but the performance of that weakest cell will be generally the limiting factor with regard to the performance of the pack as a whole. If all six cells of a 12-volt pack are equally good, drawing its voltage down to 9 may draw all six cells down to 1.5 volts without significantly damaging anything, but if one of the cells is weaker than the others, drawing the pack voltage down to 9 may result in the weak cell's voltage being drawn down to 1.0 volts while the other five cells are at 1.6. If that's done a few times, the weak cell may become even weaker, such that its voltage drops to nothing while the other cells are still at 1.8.
In cases where a multi-cell battery pack is used for purposes of storage capacity rather than current-handling ability, I would suspect that a battery pack with electronics to draw more current from stronger batteries could yield better lifetime behavior than one which simply wires cells in series, but I'm unaware of such designs being commonplace. Nonetheless, it's important to note that in multi-cell batteries, the damage caused by under-voltage conditions will increase as the difference between stronger and weaker batteries increases, and that difference will be increased as a result of such damage.