Chemistry - Why does the cyclization of open-chain glucose occur via the C5 hydroxyl group?
Solution 1:
Due to the internal ring strain the best possible conformation is a six-membered heterocycle in this case called pyranose . A five-membered heterocycle may in rare cases also be formed, called furanose. (From left to right: α-D-Glucopyranose, β-D-Glucopyranose, α-D-Glucofuranose, β-D-Glucofuranose; courtesy of wikipedia)



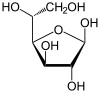
Including the linear chain form they are all isomers (wikipedia), as they have the same chemical formula. More specifically the relationship between $\ce{linear <-> pyranose <-> furanose}$ is called constitutional or structural isomer.
The $\ce{\alpha-D <-> \beta-D}$ forms are diastereomers, or more specific diastereomeric conformers.
Solution 2:
The cyclic form of glucose uses the C5 oxygen for bonding since it forms a 6 member ring (more stable then either 5 or 7 member ring).
They are isomers since they have the same chemical formula.