Why do we perceive a mixture of blue and yellow paint as green?
Color is not a physical phenomenon, it is how light of different frequencies/wavelength are perceived by humans. While the cones are developed by our animal ancestors and we share them with other species, it is entirely possible (but unlikely) that they perceive 700 nm light not a "red" as we do, but as completely different impression. What we do know is that people cannot perceive some shades at all (they are color-blind, a red-green blind person will never be able to perceive red or green) and some species and extremely rare humans can see more colors than we do, they have one additional receptor (tetrachromacy).
Back to your question.
We are only looking at the visible part of the electromagnetic waves.
If we order this part from the lowest wavelength to the highest wavelength, we see a spectrum. The curves inside the picture displays the sensitivity of the three different receptors we are using.
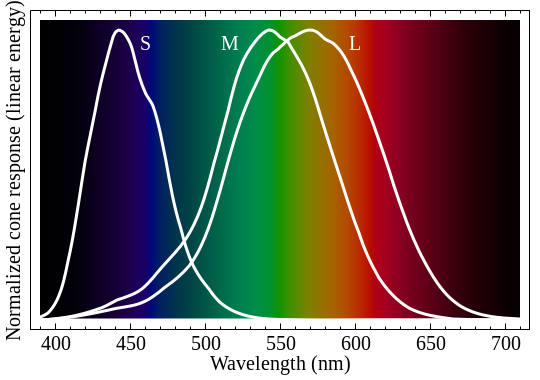
Image taken from en.wikipedia.org by BenRG, Public Domain
So light of wavelength 575 nm is perceived by our eye, stimulates both L and M receptors and our brain processes it as "yellow". But we can also use two wavelengths 540 nm and 610 nm, vary their intensity and get the exact same "yellow" impression. Principally you have a vast range of possibilities to display the exact same color.
I think I have made clear the difference between physical wavelength and perceived color. One specific wavelength always creates one color, but the same color can be created by many possible combinations of physical wavelengths.
For the sake of shortness I now define the part with the shortest wavelength as "blue" light, the part with the longest wavelength as "red" light and the middle part as "green" light. You see from the picture that you cannot define strict boundaries because the sensitivity of the receptors is overlapping. If light is completely missing, we see "black", if every component (blue, green, red) are approximately equal in intensity we call it "white".
For luminous objects color creation is easy to understand: They are sending light out and the resulting light mix is interpreted by our eyes. Monitors use light of 476, 530 and 622 nm to approximate each input.
But paint and non-luminous objects in general need light to be visible. A monitor can be seen in a dark room, but every other object is black. So the only possibility for non-luminous objects to be perceived as colorful is reflecting back some wavelengths more than others.
Let's say our object absorbs "blue" light completely and throws everything else back. I illuminate it
- with "blue" light, it seems to be black.
- with "green" light, it looks green.
- with "red" light, it looks red.
- with "white" light, the "blue" component is removed, only "red" and "green" remains...it is looking yellow.
I have now another paint which absorbs "red" light completely. I again illuminate it
- with "red" light, it seems to be black.
- with "green" light, it seems to be green.
- with "blue" light, it seems to be blue.
- with "white" light, the "red" component is removed, only "green" and "blue" remains, it is looking like cyan (a greenish blue).
A material absorbing "green" light looks purple, for the impression see the overlapping sections in the following picture. The first image shows what happens if you overlay luminous light (additive colors), the second image shows what happens if you mix paint (subtractive colors).
First image taken from en.wikipedia.org by SharkD, Public Domain; second image taken from de.wikipedia.org by Quark67 CC BY-SA 2.5
If I mix the pigments of paints, each component will absorb its wavelength component(s). In case of "blue" paint mostly "red" light is absorbed, in case of "yellow" paint mostly "blue" light is absorbed, so the dominant remaining color is green. This is the exact reason plants are looking green because plants are mainly absorbing "red" and "blue" light; plant lights are emitting therefore mainly "red" and "blue" light, the color of a plant light looks purple.
If every component is absorbed, mixing yellow, purple and cyan together should give black. In real-life you get a dark brown because the pigments are not mixing perfectly so a color tint is remaining. For that reason we use black ink in our printers for printing grey or black.
You're asking several related questions here, so let me just address the simplest one: why does a mix of blue and yellow light look green, even if there's no green in it at all?
Imagine you have a bathtub with three taps, which give out hot, warm, and cold water. If your only way of testing the temperature is to stick your hand in the water, then the output of a warm tap only feels the exact same as an equal mix of the hot and cold taps, or an equal mix of all three, etc. In particular, it can feel warm even if the warm tap is completely off!
Sight is unique among the senses because it's "unfaithful" like this.
- Sound can occupy a continuous range of frequencies. We have thousands of differently-sized hair cells that each pick out a different small frequency range.
- Smell is due to an enormous range of different molecules. We have thousands of different receptors that detect each type individually.
- Light can occupy a continuous range of frequencies. We have three different receptors, each of which are sensitive to a huge range of frequencies.
Going with the bathtub analogy, other senses are like reading the individual taps. Sight is more like testing the water. So it's no surprise that different combinations of electromagnetic waves can yield the same subjective color.
It is because your eye's green-sensitive receptors are stimulated by those colours more than are the other types of receptor.
Each of the three types of colour receptor has an overlapping curve of colour sensitivity.

From gsu.edu