Chemistry - How do I visualize an atom?
Solution 1:
I have searched and searched, oh how I have searched.
Do you know what I always tell my mom when she asks me to find something in the Internet she was not able to find herself? I ask her: "Are you sure that the thing you are looking for even exists?"
I am looking for a 3 dimensional visualization of a whole (moderately complex, hydrogen is just a ball) atom that includes 3 dimensional orbital geometry.
- Hydrogen atom is not "just a ball".
- There is no orbitals. In short, except for one-electron systems (such as the hydrogen atom) orbital description is just an approximate model of the reality.
- Usually when one asks to visualize a physical object, he/she wants to visualize its physical "boundaries" with respect to some medium. The notion of such "boundary" simply looses its meaning in the microscopic world: atoms do not have "boundaries" like macroscopic objects do.
Please. The universe is weird and I want to see.
Sorry, but you can not do this: you can not visualize some things in the Universe, you can not even imagine them. Our visualization & imagination abilities (unlike an atom) has some boundaries, because they are based ultimately on our senses which in turn has their boundaries.
As Paul Dirac said on that matter:
[…] the main object of physical science is not the provision of pictures, but is the formulation of laws governing phenomena and the application of these laws to the discovery of new phenomena. If a picture exists, so much the better; but whether a picture exists or not is a matter of only secondary importance. In the case of atomic phenomena no picture can be expected to exist in the usual sense of the word 'picture', by which is meant a model functioning essentially on classical lines. One may, however, extend the meaning of the word 'picture' to include any way of looking at the fundamental laws which makes their self-consistency obvious. With this extension, one may gradually acquire a picture of atomic phenomena by becoming familiar with the laws of the quantum theory.
Solution 2:
I am looking for a 3 dimensional visualization of a whole (moderately complex, hydrogen is just a ball) atom that includes 3 dimensional orbital geometry.
3 dimensions is only enough to represent the probability density function of hydrogen. Given that the origin is the location of the nucleus, each point in 3-dimensional space will have a corresponding probability density of the electron being at the point.
For helium, 6 dimensions would be needed to represent the probability density. This is because for each pair of points at which the nucleus and first electron are located, every point in 3-dimensional space will be associated with a probability density for the second electron. Understanding this, I would recommend the figures in 2-Particle density: Correlated motion of electrons as the next step in visualizing an atom beyond hydrogen.
Solution 3:
As a matter of fact, despite the answer you accepted, these orbitals can be visualized. The way this is done, is that an isosurface of the probability density can be plotted. That is, a surface of constant probability around the nucleus can be visualized.
There weird thing is that there is not a unique way to visualize the orbitals on the molecule. They way you choose to visualize the orbitals will depend on what kind of chemical insight you want to have.
I recommend software like Avogadro to visualize molecules.
Solution 4:
Orbitals are only mathematical functions that approximate the quantum state of an electron. Not only orbitals do not visually represent atoms, but orbitals do not have physical existence, they are only mathematical objects.
To really visualize atoms we have to use some topological definition of atom that uses electron density $\rho$, and then plot this density in 3D using some computational software.
A topological definition of atom is as a region of real space bounded by surfaces through which there is a zero flux in the gradient vector field of the electron density. Thus an interatomic surface satisfies the "zero-flux" boundary condition for every point $\mathbf{r}_s$ on the atomic surface
$$\nabla \rho(\mathbf{r}_s) \cdot \mathbf{n}(\mathbf{r}_s) = 0$$
and where $\mathbf{n}(\mathbf{r}_s)$ is the unit vector normal to the surface.
The shape of a free atom is well-represented by spherical symmetry, but atoms in molecules have very different shapes. Even the same kind of atom can have different shapes and volumes (and so different physical and chemical properties) in different molecules. Some examples for small molecules (you can check, for instance, as H atoms have different shape and volume on different molecules)
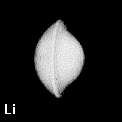

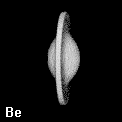

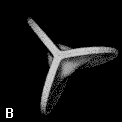
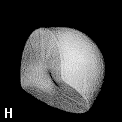

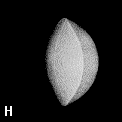

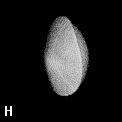

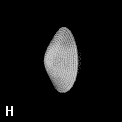

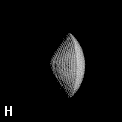
Solution 5:
Already there are sufficient answers to your question .but I want to share my opinion too ;) . Pre-note : Orbital does not means electron , what you can visualize is just where it will likely to be found which is basically an orbital .
First of all you can't visualize an orbital because it has 3 independent variables (only In case of Hydrogen like atom . for other atoms having 2 or more electrons it is greater than 3 ) , to visualize it you need a 4-D vision , which is certainly not possible. But you can visualize some of it's fraction , like 99% by using of shades. Where it is more likely to be found you can make it darker or where it is not likely be found leave it white

1s Orbital
 2s Orbital
2s Orbital
 2p Orbital
2p Orbital
There is another way but it does not look nice , the way is we enclose some consecutive volumes to describe the probability density for finding electron in respective volume (Something like layers ) (The right image is the Contour plot, the left one is the same as above ).

If you move on any line , you will find yourself on a area where probability density is same . These are called isosurfaces . These can be very fancy , nice to visualize . Here are some Iso Boundary Surfaces of some orbitals .
Caution : Any of these images does not show images of orbitals , these are only Boundary Surfaces . (In many books these pictures are referred to Images of orbital without any clarification which can be misleading )

